Single-Cell Signalling Analysis of Tumour Microenvironment Chemoradiotherapy Protection in Rectal Cancer
Primary supervisor: Chris Tape, UCL
Secondary supervisor: Vivian Li, Francis Crick Institute
Project
Colorectal cancer (CRC) is the third most common cancer in the UK and its incidence is increasing (>42,000 cases/ year between 2016 and 2018). Rectal cancer accounts for ~30% of CRC and has a worse prognosis than colon cancer. Treatment options differ for colonic and rectal cancers, prompting the need to study these diseases separately.
The varying response of locally advanced rectal cancer (LARC) to neoadjuvant chemoradiotherapy (nCRT) presents an ongoing challenge for management and prognosis. Cancer-associated fibroblasts (CAFs) in the tumour microenvironment (TME) have been implicated in cancer progression and response to drug therapy. However, CAF-mediated protection of rectal cancer to (nCRT) has not been mechanistically explored.
Through this CRUK City of London Centre Research Training Fellowship, we will explore the mechanisms in which CAFs regulate the response of rectal cancer to nCRT. We will co-culture patient-derived organoids (PDOs) from rectal cancer, with patient-matched CAFs to model the TME. We will use Thiol-reactive Organoid Barcoding in situ (TOBis) mass cytometry (MC), a technology recently developed by the Tape lab (Qin et al., Nature Methods, 2020 and Sufi & Qin et al., Nature Protocols, 2021), to analyse intra- and inter-cellular signalling via >30 post-translational modifications (PTMs) at single-cell resolution across hundreds of PDO- CAF co-cultures.
Aims:
- Quantify rectal PDO-CAF signalling after nCRT.
- Compare PDO-CAF signalling after nCRT in vivo vs in vitro.
- Manipulate PDO-CAF signalling to improve nCRT response.
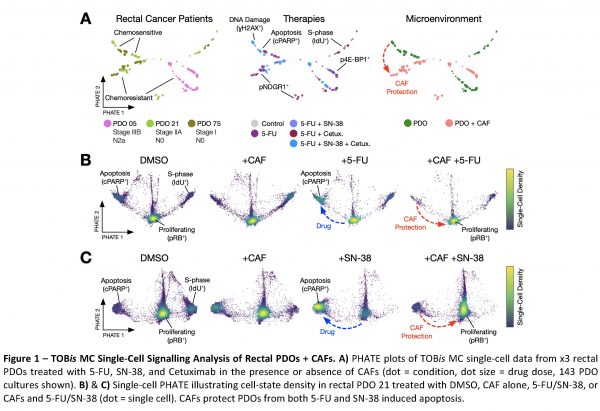
Preliminary TOBis MC data from the Tape lab has shown that CAFs protect chemosensitive rectal PDOs from 5- fluorouracil (5-FU) and SN-38 (irinotecan) chemotherapies (Figure 1). CAFs stimulate PTM signalling in PDOs (e.g. pNDRG1 and pGSK-3β) which in turn protect cancer cells from chemotherapy induced apoptosis. Several dysregulated PTM pathways are patient-specific, whereas others which are shared between patients.
PDOs and CAFs will be derived from nCRT-naïve specimens using an established pipeline at University College London Hospital (UCLH). PDOs will be given nCRT alone or in co-culture with CAFs. Mitogenic, cell-cycle, DNA- damage, and apoptotic PTMs will be analysed at single-cell resolution. PDOs and CAFs will also be derived from post-nCRT specimens to compare PDO-CAF signalling after in vitro and in vivo nCRT.
Targeting mechanisms of CAF-mediated regulation of nCRT response may have a profound impact on prognosis in LARC. Achieving these aims would allow us to subsequently explore if PDOs from patients before they receive nCRT can be exploited as predictive avatars to develop patient-specific treatment strategies.
Candidate background
This project is particularly well suited to a candidate with experience in organoid culture, intestinal cancers, radiotherapy, and/or single-cell analysis. Candidates with clinical experience treating rectal cancer with radiotherapy are particularly encouraged to apply.
References
- Sufi & Qin et al., Nature Protocols, 2021.
- Qin et al., Nature Methods, 2020.
- Tiriac et al., Cancer Discovery, 2018.
- Isella et al., Nature Genetics, 2015.
