Targeted Platinum-based Chemotherapy for the Treatment of Neuroblastoma
Primary supervisor: Mahvash Tavassoli, King’s College London
Secondary supervisor: Stefano Giuliani, UCL
Tertiary supervisor: Adam Sedgwick, King’s College London
Project
Summary: Hypoxia (low oxygen) is a common characteristic of most solid tumours. Exploiting tumour hypoxia to convert a “non-toxic” prodrug into the active toxic counterpart has the potential to afford a cancer selective therapeutic and overcome the dose limiting effects of traditional chemotherapy.
Within most solid tumours, there are regions of hypoxia (low oxygen levels) due to the increased metabolic demand of cancer cells and poor tumour vasculature [1]. The severity of hypoxia can vary between tumour types. Importantly, several studies in a range of tumour types have demonstrated that hypoxia correlates with poor patient prognosis and increased tumour aggressiveness, therefore making tumour hypoxia a key therapeutic target within oncology [1,2]. The significant differences in oxygen levels between tumour and healthy tissue provide an avenue to exploit for the development of a cancer-selective therapy. We have shown that hypoxia plays an important role in treatment resistance of head and neck cancers [2]. Considerable research efforts have been made towards developing hypoxia-activated prodrugs (HAPs). HAPs are compounds “masked” with protecting groups that are designed to be selectively removed under hypoxic conditions to form the corresponding cytotoxic drug. Current reported HAPs have utilised quinone, nitroaromatic, and N-oxide-based protecting groups to mask cytotoxic drugs. Several HAPs have entered clinical trials (e.g., TH-302, TH-4000) and to date, several companies are focusing on the clinical development of HAPs such as Convert Pharmaceuticals and OncoTherics [1].
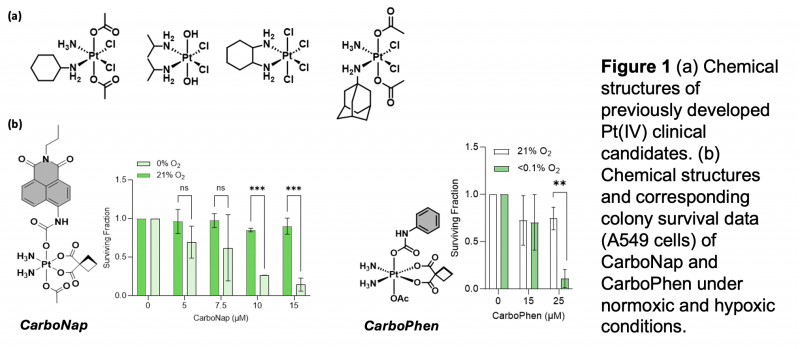
In this project we propose to develop hypoxia-mediated activation of Pt(IV) prodrug into the active toxic Pt(II) counterpart (Figure 1). Several hypoxic cancers are treated with a Pt(II) chemotherapy; therefore by designing a HAPt, we have the potential to selectively deliver the beneficial therapeutic properties of Pt(II) while overcoming the dose-limiting off-target toxicity [3,4].
We will test our compounds in neuroblastoma (NB); a highly malignant paediatric solid tumour developing from neural-crest-derived cells during foetal or early postnatal life, is one example in which a hypoxic signature is associated with dismal patient outcome. Hypoxia is known to trigger dedifferentiation of NB cells towards a more immature stem cell phenotype and is associated with NB metastasis. More than 60% of NB tumours are metastatic and secondary tumours can be found in bone, bone marrow, liver, lymph nodes or, less commonly, in the skin, lung or brain. To successfully treat these young patients, the solid tumour must be removed completely. However, the surgery to remove neuroblastoma is challenging due to the fact that vital organs, vessels and nerves are often enchased by the tumour. To avoid major patient complications, incomplete resection is often the surgical outcome and patients are given chemotherapy either systemically or through electrochemotherapy (ECT) (studies carried out in the group of supervisor SG).
Here, we propose to carry out a comprehensive structure-activity relationship study to help develop lead Hypoxia Activated Pt(IV) (HAPt) using a Pt(IV) complex modified with a fluorescent reporter (PlatFluors) (Fig1). We would test the previously developed clinical candidates as well i.e. Satraplatin – (Fig1a). Fluorescent PlatFluors that mimic the design of a HAPt will provide the rapid ability to identify and predict the best chemical design/motif for the development of lead candidates for potential clinical translation.
Specific objectives are:
- Objective 1: Synthesise and validate fluorogenic Pt(IV) complexes (PlatFluors) and to develop the methodology to activate carboplatin-based prodrugs in cells.
- Objective 2: Assess the hypoxia signalling pathways in 2D and 3D models of NB under hypoxic and normoxic conditions.
- Objective 3: Validate the optimised methodology in established neuroblastoma cell lines, spheroids, organoids and animal models at GOSH/ UCL. GOSH/UCL team holds the animal licence and have the facilities for the required animal experiments. We will combine both fluorescence and viability assays in this objective.
Candidate background
The candidate is expected to have a first degree in biological sciences, related fields or a medical degree. As the project involves chemical synthesis of probes, knowledge of chemistry would be desirable but not necessary as comprehensive training will be provided in the conceptual and technical aspects.
Potential Research Placements
- Adam Sedgwick, Department of Chemistry, King’s College London
- Stefano Giuliani, Great Ormond Street Hospital/ Institute fir Child Health, UCL
- Ismael Díez-Pérez, Department of Chemistry, King’s College London
References
- Codony VL, Tavassoli M. Hypoxia-induced therapy resistance: Available hypoxia-targeting strategies and current advances in head and neck cancer. Transl Oncol. 2021;14(3):101017.
- Suh, Y-E., Lawler, K. J., Henley-Smith, R., Pike, L., Leek, R., Barrington, S. F., Odell, E. W., Ng, T. T., Pezzella, F., Guerrero-Urbano, M. T. & Tavassoli, M. (2017). Association between hypoxic volume and underlying hypoxia-induced gene expression in oropharyngeal squamous cell carcinoma (OPSCC): Hypoxia biomarkers from 64Cu-ATSM PET/CT imaging., BJC, 116, p. 1057?1064
- Huang S, Marsh JW, White JRG, et al. A colorimetric approach for monitoring the reduction of platinum(iv) complexes in aqueous solution. New J Chem. 2024;48(17):7548-7551. Published 2024 Apr 10.
- Boulet MHC, Bolland HR, Hammond EM, Sedgwick AC. Oxali(IV)Fluors: Fluorescence Responsive Oxaliplatin(IV) Complexes Identify a Hypoxia-Dependent Reduction in Cancer Cells. J Am Chem Soc. 2023;145(24):12998-13002.
- Fardin P, Barla A, Mosci S, et al. A biology-driven approach identifies the hypoxia gene signature as a predictor of the outcome of neuroblastoma patients. Mol Cancer. 2010;9:185. Published 2010 Jul 12. doi:10.1186/1476-4598-9-185
