A translational study of the role of Fusobacterium in oral cancer treatment efficacy using tumour-on-chip models
Primary supervisor: Miguel Reis Ferreira, King’s College London
Secondary supervisor: Oliver Pearce, Queen Mary University of London
Tertiary supervisor: Stefaan Verbruggen, Queen Mary University of London
Project
Oral squamous cell carcinoma (OSCC) is devastating. Patients with high-risk OSCC undergo mutilating surgery and morbid post-operative radiotherapy (+- chemotherapy). Despite this, recurrence rates are high. Relapsed OSCC is notoriously difficult to treat. There is a clinical unmet need to improve OSCC treatment, which has not significantly changed in the last decade.
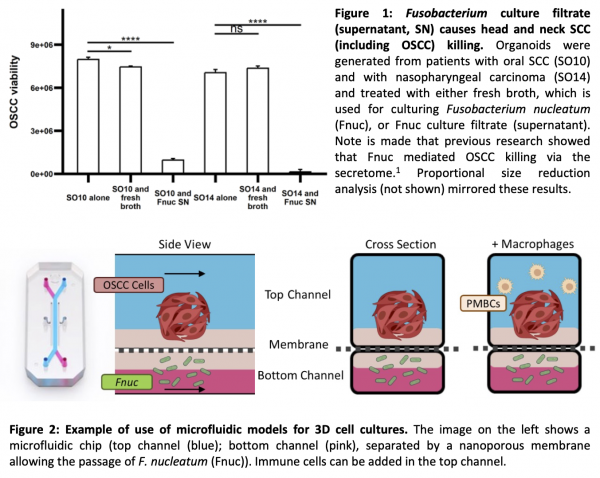
The KCL Reis Ferreira (MRF) group has identified that patients with detectable and/or higher abundance of Fusobacterium, a genus of Gram-negative anaerobic oral commensal bacteria, in their OSCC have better survival and that these bacteria promote OSCC death in 2D (published) and 3D (figure 1) cell culture models [1]. Published research also indicates that Fnuc impacts on cancer-associated immunity in colorectal and oral cancers [2,3].
Herein, the hypothesis that tumour-associated Fnuc improves the effectiveness of non-surgical OSCC treatment will be tested.
The candidate will initially develop innovative 3D tumour-on-chip microfluidic models of OSCC, immune cells and bacteria (figure 2) at the QMUL Pearce and Verbruggen labs, specialising in tumour microenvironment and modelling microenvironments on in vitro microfluidics and chip devices respectively [4]. OSCC 3D cultures will leverage experience from Dr Anthony Kong’s lab (KCL).
Fusobacterium strains and OSCC cell lines will be used for optimisation. An irradiation protocol using clinically-active linear accelerators will be developed leveraging previous experience of MRF’s group [5].
Using tissue from patients treated for stage III-IV OSCC, Fusobacterium communities will be characterised via deep sequencing and their spatial localisation defined using RNAscope. Organoids will be generated, as will matched Fusobacterial culture collections and PBMCs. The radiosensitivity of patient-derived organoids will be ascertained and the effect of Fusobacterium co-culture in radiotherapy effectiveness evaluated. Proteomics and phosphoproteomics will delineate differential expression in OSCC treated with combined Fusobacterium and radiotherapy.
T and NK cells will be purified from PBMCs. T cell immunoreceptor with Ig and ITIM domains (TIGIT), which interacts with the Fusobacterial Fap2 protein, will be quantified with flow cytometry, as will Programmed Death-1 (PD1). Programmed Death ligand-1 will be quantified in OSCC and differential expression quantified in organoids after treatment with Fusobacterium. After mechanistic delineation, combined radio-immunotherapy will be tested in the presence or absence of Fusobacterium to assess whether it potentiates combined treatment.
Candidate background
This project would suit candidates with a background in biology and an interest in cancer and/or radiotherapy research, 3D and organ-on-chip models.
Potential Research Placements
- Rebecca Carter, Preclinical Radiotherapy Core Facility, UCL
- David Moyes, Centre for Host-Microbiome Interactions, King’s College London
- Aleksey Chudnovskiy, Francis Crick Institute
References
- Chander, A. et al. Fusobacterium is toxic for head and neck squamous cell carcinoma and its presence may determine a better prognosis. Cancer Commun (2024) doi:10.1002/cac2.12588.
- Michikawa, C. et al. Fusobacterium is enriched in oral cancer and promotes induction of programmed death-ligand 1 (PD-L1). Neoplasia 31, 100813 (2022).
- Gur, C. et al. Binding of the Fap2 protein of fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity (2015) doi:10.1016/j.immuni.2015.01.010.
- Nolan, J., Pearce, O. M. T., Screen, H. R. C., Knight, M. M. & Verbruggen, S. W. Organ-on-a-Chip and Microfluidic Platforms for Oncology in the UK. Cancers (Basel) 15, 635 (2023).
- Chander, A. et al. PO-2213 The impact of the tumour microenvironment on head and neck SCC cell viability and radiosensitivity. Radiotherapy and Oncology 182, S1991-S1992 (2023).
