Imaging Patient Therapy Response
The aim of our research is to evaluate response to conventional or novel treatments using ex vivo patient-derived models (tumour slices and patient-derived organoids).
Using imaging multi-photon confocal technology and custom-designed equipment we can analyse parameters such as lymphocyte infiltration, cell death and cell proliferation and prognosticate the patient’s response or resistance to the suggested treatment, therefore leading to a better stratification.
Our efforts are currently focused in colon adenocarcinoma, head and neck carcinoma and neuroblastoma research but we are open to new collaborations.
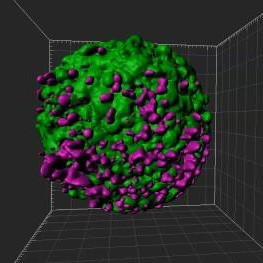
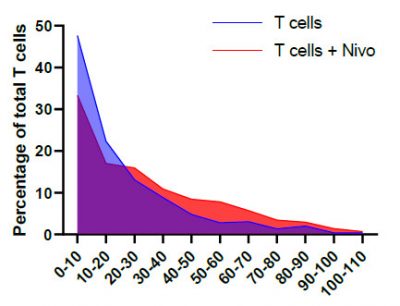
A breast cancer spheroid (green) being penetrated by fluorescently labelled T lymphocytes (magenta) that have been pre-treated with anti-PD-1 (Nivolumab). A quantifiable increase in immune cell penetration following Nivolumab treatment was detected by comparing the histograms of T cell infiltration distances.
Contact
Academic Lead
Ongoing projects/collaborations
Collaborators: Tony Ng (UCL/KCL), Daniel Hochhauser (UCL), Manuel Rodriguez Justo (UCL/UCLH) and Paul Barber (UCL).
Colorectal cancer (CRC) is one of the most common malignancies and presents limited treatment options in the metastatic setting. Until recently, conventional chemotherapy such as FOLFOX or FOLFIRI formulated the backbone of treatment for all cases with modest response rates. Targeted therapies including the epidermal growth factor receptor (EGFR) monoclonal antibodies cetuximab and panitumumab, and immune checkpoint inhibitors have also been incorporated recently.
The objectives of the study focus on gaining a better understanding on the impact of EGFR-targeted therapy (cetuximab), immunotherapy (nivolumab) and conventional chemotherapy on immune (T-cell) infiltration. By assessing that in patient-derived organoids, we expect the knowledge to be rapidly translated into the clinical setting.
The potential translational impacts of the project are to prognosticate the patient response to cetuximab and chemotherapy treatment and evaluate the potential additional therapeutic benefits of combining immune checkpoint inhibitors with cetuximab and chemotherapy in a patient stratification algorithm based on organoid imaging.
Collaborators: Tony Ng (UCL/KCL), Martin Forster (UCLH), Sergio Quezada (UCL) and Fran Balkwill (QMUL).
Head and neck cancer (HNSCC) is the 8th most common cancer in the UK, with 12,000 new cases diagnosed yearly. Common treatment includes chemo- and radiotherapy following surgical resection. There is no standardised programme for surveillance, and practice varies widely between hospitals and clinicians leading to inconsistencies. More importantly, existing surveillance programmes are unable to adapt to the different risks of cancer recurrence in individual patients, because of a lack of understanding of the factors that influence the risk of recurrence in different patients.
By using a novel imaging approach, ex vivo tumour slices obtained from patients are being subjected to treatment, aiming to extract key information, such as changes in the architecture of tumour, distribution and types of immune cells, and even bacteria and their influence on the immune cells, in response to their initial treatment. We will then use a complex model to integrate these individual variables and link them in order to predict how well patients will respond to treatment, and the likelihood for cancer recurrence.
Collaborators: John Anderson (ICH/UCL), Jane Sosabowski (QMUL) and Tony Ng (UCL/KCL).
Neuroblastoma is the most common solid tumour affecting children. Despite improvements, around 50% of high risk patients relapse or fail to respond, and outcomes remain poor. Chemotherapy resistance is the major limitation in high risk neuroblastoma, and local surgical or radiotherapy strategies are therefore not curative. Immunotherapy (CAR-T cells) has recently emerged as an attractive modality.
We aim to evaluate the effect of low dose radiation on the capacity of fluorescently-labelled CAR-T cells (using GD2 as a model antigen) to infiltrate patient-derived neurosphere models and overcome immune suppression.
Additional information
- Tumour slice protocol: https://www.frontiersin.org/articles/10.3389/fimmu.2015.00500/full
- Tissue clearing protocol: https://onlinelibrary.wiley.com/doi/abs/10.1002/lpor.201800292