Targeting macrophage lipid metabolism to accelerate skin repair during radiotherapy for breast and head and neck cancers
Primary supervisor: Clare Bennett, UCL
Secondary supervisor: Patricia Barral, King’s College London
Project
Radiotherapy is a mainstay of cancer therapeutics but damage to healthy tissue remains the major factor limiting use; in breast and head and neck cancers, acute and chronic skin damage with severe fibrosis at the radiation site are major adverse events, restricting treatment and imposing poor life quality. Finding approaches for prevention and management of radiation-induced skin toxicities will improve clinical outcomes for cancer patients.
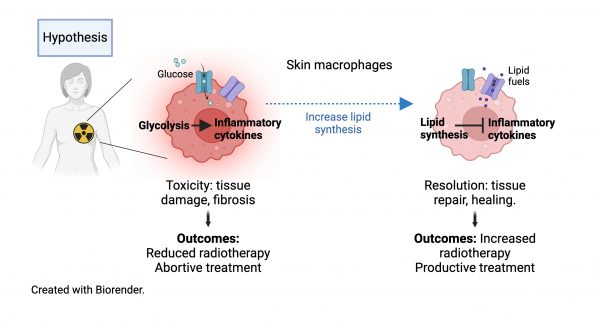
Macrophages are the predominant immune cells in the skin and attractive targets to heal radiotherapy-induced damage due to their unique tissue repair functions. This capability is driven by lipid metabolic programming that switches off pro-fibrotic inflammatory pathways, turning on a tissue restoration state. Recent data from our labs identified new molecular pathways controlling lipid metabolism in macrophages [1,2] and show the importance of lipid metabolism and biosynthesis for the recovery of skin macrophages after total body irradiation [3]. But pro-fibrotic macrophages persist in the skin post-irradiation leading to long-term “immunological scarring” [4].
Our aim is to determine how radiotherapy alters skin macrophage states and elucidate mechanisms to accelerate skin healing. We hypothesize that manipulating lipid metabolism to activate tissue repair by macrophages will provide a new approach for protecting healthy tissue from radiation-induced toxicity and enhance treatment efficacy. To test this, we have defined 3 aims:
Aim 1. To define normal tissue macrophage responses and the impact of different treatment parameters (radiation field, dose, fractionation scheme), we will use targeted X-ray irradiation at the pre-clinical radiation research platform (UCL Cancer Institute). Combining histology and proteome profiling will determine extent of skin changes, kinetics of resolution, and production of key radiotherapy-induced pro-fibrotic factors (TGFb, PDGF) in skin. Phenotypic and metabolic analyses of macrophages by flow-cytometry and approaches for ex vivo metabolic profiling will define activation dynamics of cutaneous macrophage populations switching from inflammatory (pro-fibrotic) to resolution states.
Aim 2. To determine mechanisms by which activation of lipid metabolism promotes skin repair after radiation we will sequence the transcriptomes and measure the lipidome of cutaneous macrophages at different time points. Integration of the datasets will identify lipid pathways and species associated with tissue-repair macrophage functions. Cross-reference to the Human Skin Atlas will determine conserved pathways. In vitro experiments with specific lipids/inhibitors will identify candidates for further testing in vivo.
Aim 3. To demonstrate improved clinical efficacy, we will topically apply radioprotective lipids/lipid metabolism activators in pre-clinical models of orthotopic breast cancer (E0771) or head and neck cancer cells (JC1-2) to model residual disease. Outcomes will be skin toxicity (including impacts of surgery), kinetics of tissue repair and tumour regrowth. We predict that skin protection will permit use of higher doses and/or more frequent application of radiotherapy to prevent tumour-re-growth. Professor Hodivala-Dilke has these models established in her lab [5] and will support this aim.
Candidate background
This project would suit motivated candidates with strong backgrounds in cancer/skin biology or immunology. They will receive comprehensive training in wet lab techniques (flow cytometry, imaging, metabolic assays) computational metabolomics/transcriptomics, and pre-clinical tumour models.
Potential Research Placements
- James Macrae, Metabolomics Facility, Francis Crick Institute
- Maria Secrier, Genetics Institute, UCL
- Kairbaan Hodivala-Dilke, Barts Cancer Institute, Queen Mary University of London
References
- Brailey et al. CD1d-dependent rewiring of lipid metabolism in macrophages regulates innate immune responses (2022) Nature Communications doi.org/10.1038/s41467-022-34532-x (Barral lab)
- Sáez de Guinoa et al. CD1d-mediated lipid presentation by CD11c+ cells regulates intestinal homeostasis (2018) EMBOJ DOI: 10.15252/embj.201797537 (Barral lab)
- Appios et al. Convergent evolution of monocyte differentiation in adult skin instructs Langerhans cell identity. (2023) BioRxiv doi.org/10.1101/2023.11.13.566862. (Bennett lab)
- West et al. Loss of T cell tolerance in the skin following immunopathology is linked to failed restoration of the dermal niche by recruited macrophages (2022) Cell Reports doi: 10.1016/j.celrep.2022.110819. (Bennett lab)
- Wong et al. Cancer Burden Is Controlled by Mural Cell β3-Integrin Regulated Crosstalk with Tumor Cells (2020) Cell DOI: 10.1016/j.cell.2020.02.003 (Hodivala-Dilke lab).
