Understanding B Cell Dynamics and ECM Topography in Melanoma Through Multiomic Analysis and Predictive Modelling of Risk Signatures
Primary supervisor: Oscar Maiques, Queen Mary University of London
Secondary supervisor: Sophia Karagiannis, King’s College London
Project
B cells play crucial roles in melanoma immune surveillance and may significantly influence cancer progression and treatment outcomes [1]. Despite their importance, the roles of B cells in melanoma are still not well understood, necessitating further research to elucidate their functions and contributions. Amoeboid cancer cells at the invasive front of melanoma exhibit high-actomyosin contractility and hijack immune cells like macrophages to create a tumour-promoting environment [2,3]. This interaction enhances drug resistance, stemness [4], and tumour progression, highlighting the need for targeted therapeutic strategies [5]. Unpublished work by our group shows that matrix alignment significantly influences melanoma dissemination, with increased alignment and radial orientation at the distal invasive fronts (DIF) compared to proximal fronts or the tumour body (Maiques et al., unpublished). These findings highlight the ECM architecture’s potential as a biomarker for invasion and poor prognosis in advanced melanoma. Furthermore, research in the Karagiannis laboratory has revealed that B cells differentiate, evolve and clonally expand across different compartments of the tumour microenvironment, including tumour islands, stroma and tertiary lymphoid structures (TLS) [1]. Additionally, studying the interactions between B cells and ECM structures may reveal novel biomarkers for predicting risk progression and therapeutic outcomes in melanoma patients.
Description and Aims
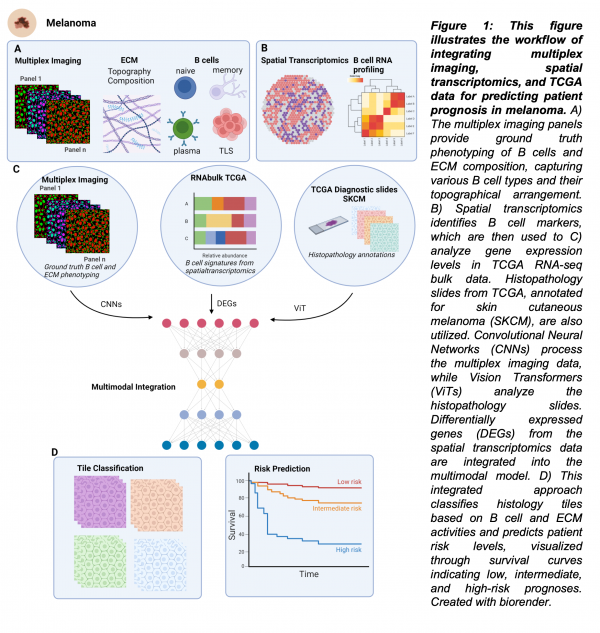
We aim to understand the role of B cell dynamics in melanoma progression through a multidisciplinary approach. Initially, by employing Multiplex Cell Dive technology, we will, 1) investigate the relationship between extracellular matrix (ECM) architecture and B cell behaviour during melanoma progression. This will allow us to visualize ECM components and B cell distribution in melanoma tissues, analysing ECM remodelling and correlation with B cell infiltration and identification of TLS (Figure 1A).
Next, 2) utilize spatial transcriptomics data from primary and regional melanoma metastases samples to create detailed signatures of B cell states at the RNA level. This involves identifying and validating B cell-specific gene expression signatures and mapping these to spatially resolved data to understand cell localization and function (Figure 1B), 3) develop deep learning models to predict B cell presence, subtype, and function in histology images using TCGA Skin Cutaneous Melanoma (SKCM) samples. This will involve preprocessing and annotating histology images, training models with bulk RNA-seq data, and validating predictions with independent datasets (Figure 1C). Finally, 4) we will assess the prognostic value of predicted B cell activity in stratifying for overall survival (Figure 1D). A critical aspect of this project is to determine whether ECM architecture or composition contributes to B cell dynamics, particularly TLS formation. This connection could provide significant insights into how ECM remodelling influences B cell behaviour and melanoma progression.
Candidate background
A potential candidate should have a Master’s degree in bioinformatics or machine learning. Knowledge in pathology, cancer biology and immunology is recommended. They should possess knowledge of advanced imaging techniques, such as multiplex imaging and segmentation tools, and experience in analysing high-throughput sequencing data, including RNA-seq and spatial transcriptomics (desired). Familiarity with machine learning (ML) and computer vision (CV) domains, particularly deep learning applied to biological datasets, is highly desirable. The candidate should have strong analytical skills, proficiency in programming languages like Python or R.
Potential Research Placements
- Sophia Karagiannis, St John’s Institute of Dermatology, King’s College London
- Claude Chelala, Barts Cancer Institute, Queen Mary University of London
- Vivek Singh, Barts Cancer Institute, Queen Mary University of London
References
- Crescioli, S., Correa, I., Ng, J., Willsmore, Z.N., Laddach, R., Chenoweth, A., Chauhan, J., Di Meo, A., Stewart, A., Kalliolia, E., … Karagiannis SN. (2023). B cell profiles, antibody repertoire and reactivity reveal dysregulated responses with autoimmune features in melanoma. Nature Communications 2023 14:1 14, 1-21. https://doi.org/10.1038/s41467-023-39042-y.
- Georgouli, M., Herraiz, C., Crosas-Molist, E., Fanshawe, B., Maiques, O., Perdrix, A., Pandya, P., Rodriguez-Hernandez, I., Ilieva, K.M., Cantelli, G., … Karagiannis SN, Sanz-Moreno V. (2019). Regional Activation of Myosin II in Cancer Cells Drives Tumor Progression via a Secretory Cross-Talk with the Immune Microenvironment. Cell 176. https://doi.org/10.1016/j.cell.2018.12.038.
- Samain, R., Maiques, O., Monger, J., Lam, H., Candido, J., George, S., Ferrari, N., Kohihammer, L., Lunetto, S., Varela, A., et al. (2023). CD73 controls Myosin II-driven invasion, metastasis, and immunosuppression in amoeboid pancreatic cancer cells. Sci Adv 9. https://doi.org/10.1126/SCIADV.ADI0244.
- Rodriguez-Hernandez, I., Maiques, O., Kohlhammer, L., Cantelli, G., Perdrix-Rosell, A., Monger, J., Fanshawe, B., Bridgeman, V.L., Karagiannis, S.N., Penin, R.M., et al. (2020). WNT11-FZD7-DAAM1 signalling supports tumour initiating abilities and melanoma amoeboid invasion. Nat Commun 11. https://doi.org/10.1038/s41467-020-18951-2.
- Maiques, O., Fanshawe, B., Crosas-Molist, E., Rodriguez-Hernandez, I., Volpe, A., Cantelli, G., Boehme, L., Orgaz, J.L., Mardakheh, F.K., Sanz-Moreno, V., et al. (2021). A preclinical pipeline to evaluate migrastatics as therapeutic agents in metastatic melanoma. Br J Cancer 125. https://doi.org/10.1038/s41416-021-01442-6.
